-
MgxMn(1−x)(BH4)2 (x = 0–0.8), a cation solid solution in a bimetallic borohydride
R. Cerny, N. Penin, V. D'Anna, H. Hagemann, E. Durand and J. Ruzicka
Acta Materialia, 59 (13) (2011), p5171-5180


DOI:10.1016/j.actamat.2011.04.052 | unige:16759 | Abstract | Article HTML | Article PDF
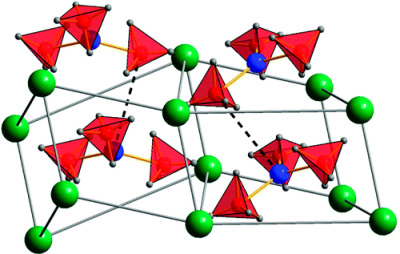
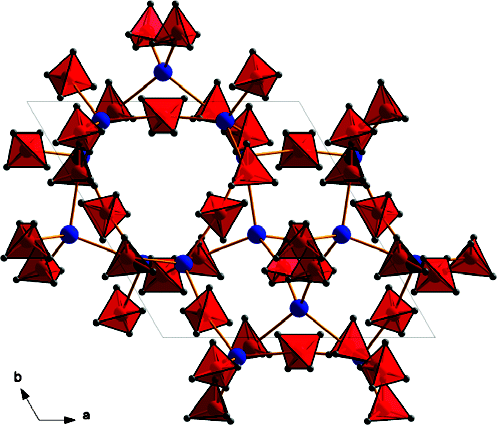
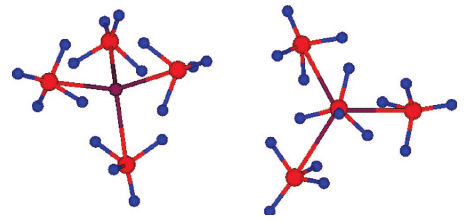
A solid solution of magnesium and manganese borohydrides was studied by in situ synchrotron radiation X-ray powder diffraction and infrared spectroscopy. A combination of thermogravimetry, mass and infrared spectroscopy, and atomic emission spectroscopy were applied to clarify the thermal gas desorption of pure Mn(BH4)2 and a solid solution of composition Mg0.5Mn0.5(BH4)2. MgxMn(1−x)(BH4)2 (x = 0–0.8) conserves the trigonal structure of Mn(BH4)2 at room temperature. Manganese is dissolved in the hexagonal structure of α-Mg(BH4)2, with the upper solubility limit not exceeding 10 mol.% at room temperature. There exists a two-phase region of trigonal and hexagonal borohydrides within the compositional rangex = 0.8–0.9 at room temperature. Infrared spectra show splitting of various vibrational modes, indicating the presence of two cations in the trigonal MgxMn(1−x)(BH4)2 solid solutions, as well as the appearance of a second phase, hexagonal α-Mg(BH4)2, at higher magnesium contents. All vibrational frequencies are shifted to higher values with increasing magnesium content. The decomposition temperature of the trigonal MgxMn(1−x)(BH4)2 (x = 0–0.8) does not vary significantly as a function of the magnesium content (433–453 K). The desorbed gas contains mostly hydrogen and 3–7.5 mol.% diborane B2H6, as determined from analyses of the Mn(BH4)2 and Mg0.5Mn0.5(BH4)2 samples. An eutectic relation between α-Mg(BH4)2 and LiBH4 is observed. The solid solution MgxMn(1−x)(BH4)2 is a promising material for hydrogen storage as it decomposes at a similar temperature to Mn(BH4)2, i.e. at a much lower temperature than pure Mg(BH4)2 without significantly losing hydrogen weight capacity thanks to substitution of Mn by Mg up to 80 mol.%. The questions of diborane release and reversibility remain to be addressed.